Molecule definition, the smallest physical unit of an element or compound, consisting of one or more like atoms in an element and two or more different atoms in a compound. A molecule is an electrically neutral group of two or more atoms held together by chemical bonds. Molecules are distinguished from ions by their lack of electrical charge. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and molecule is often used when referring to polyatomic ions. The significant difference between atom and molecule is that an atom is regarded as the tiniest particle that constitutes matter. On the contrary, a molecule is the combination of two or even more smallest units i.e., atoms that are chemically bonded together. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, as with two atoms in the oxygen molecule (O 2); or it may be heteronuclear, a chemical compound composed of more than one element, as with water (two hydrogen atoms and one oxygen atom; H 2 O).
- Atom Molecule Model
- Atom Molecule Image
- Atom Molecule Compound
- Atom Molecule Order
- Atom Molecule Ion
- Atoms And Molecules Quizlet
FREE K-12 standards-aligned STEM
curriculum for educators everywhere!
Find more at TeachEngineering.org.
Quick Look
Grade Level: 6 (5-7)
Time Required: 30 minutes
Expendable Cost/Group: US $1.50
Group Size: 2
Activity Dependency: None
Subject Areas: Chemistry, Physical Science
Summary
Students use gumdrops and toothpicks to make lithium atom models. Using these models, they investigate the makeup of atoms, including their relative size. Students are then asked to form molecules out of atoms, much in the same way they constructed atoms out of the particles that atoms are made of. Students also practice adding and subtracting electrons from an atom and determining the overall charges on atoms.Engineering Connection
Engineers and scientists have the ability to not only change one element into another, but also to make elements that do not already exist in nature. The last few elements on the periodic table are all human-made. Engineers can use these elements to serve a specific design purpose, such as making a stronger metal alloy for a bridge or building, or designing a new medicine. All types of advanced technologies are possible because engineers study the physical and chemical properties of the atom to alter their natural properties.
Learning Objectives
After this activity, students should be able to:
- List the basic components and structure of the atom.
- Identify the electrical charge of an atom and its subatomic particles.
- Explain how engineers use their knowledge of atoms to create new technologies.
Educational Standards Each TeachEngineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
Each TeachEngineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards.
All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN), a project of D2L (www.achievementstandards.org).
In the ASN, standards are hierarchically structured: first by source; e.g., by state; within source by type; e.g., science or mathematics; within type by subtype, then by grade, etc.
NGSS: Next Generation Science Standards - Science
| NGSS Performance Expectation | ||
|---|---|---|
MS-PS1-1. Develop models to describe the atomic composition of simple molecules and extended structures. (Grades 6 - 8) Do you agree with this alignment? Thanks for your feedback! | ||
| Click to view other curriculum aligned to this Performance Expectation | ||
| This activity focuses on the following Three Dimensional Learning aspects of NGSS: | ||
| Science & Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Develop a model to predict and/or describe phenomena. Alignment agreement: Thanks for your feedback! | Substances are made from different types of atoms, which combine with one another in various ways. Atoms form molecules that range in size from two to thousands of atoms. Alignment agreement: Thanks for your feedback! Solids may be formed from molecules, or they may be extended structures with repeating subunitsAlignment agreement: Thanks for your feedback! | Time, space, and energy phenomena can be observed at various scales using models to study systems that are too large or too small. Alignment agreement: Thanks for your feedback! |
Common Core State Standards - Math
- Understand that positive and negative numbers are used together to describe quantities having opposite directions or values (e.g., temperature above/below zero, elevation above/below sea level, credits/debits, positive/negative electric charge); use positive and negative numbers to represent quantities in real-world contexts, explaining the meaning of 0 in each situation. (Grade 6) More Details
Do you agree with this alignment? Thanks for your feedback!
International Technology and Engineering Educators Association - Technology
- Knowledge gained from other fields of study has a direct effect on the development of technological products and systems. (Grades 6 - 8) More Details
Do you agree with this alignment? Thanks for your feedback!
State Standards
Colorado - Math
- Explain why positive and negative numbers are used together to describe quantities having opposite directions or values. (Grade 6) More Details
Do you agree with this alignment? Thanks for your feedback!
- Use positive and negative numbers to represent quantities in real-world contexts, explaining the meaning of 0 in each situation. (Grade 6) More Details
Do you agree with this alignment? Thanks for your feedback!
Colorado - Science
- Use the particle model of matter to illustrate characteristics of different substances (Grade 6) More Details
Do you agree with this alignment? Thanks for your feedback!
- All matter is made of atoms, which are far too small to see directly through a light microscope. Elements have unique atoms and thus, unique properties. Atoms themselves are made of even smaller particles (Grade 6) More Details
Do you agree with this alignment? Thanks for your feedback!
- Identify evidence suggesting that atoms form into molecules with different properties than their components (Grade 6) More Details
Do you agree with this alignment? Thanks for your feedback!
Atom Molecule Model
Materials List
Each group needs:
- 4 red gumdrops
- 3 green gumdrops
- 3 blue/purple/white gumdrops
- 4-5 wooden toothpicks
- 3 long wooden skewers
- 10 small sticker dots
Note: Non-sugarcoated gumdrops work better for this activity. If sugarcoated gumdrops are used, students will need to use staples to attach the dot stickers to the gumdrops.
Worksheets and Attachments
Visit [www.teachengineering.org/activities/view/cub_mix_lesson1_activity1] to print or download.More Curriculum Like This
The Building Blocks of MatterStudents learn about atoms and their structure (protons, electrons, neutrons) — the building blocks of matter. They see how scientific discoveries about atoms and molecules influence new technologies developed by engineers.
Students learn about the basics of molecules and how they interact with each other. They learn about the idea of polar and non-polar molecules and how they act with other fluids and surfaces. Students acquire a conceptual understanding of surfactant molecules and how they work on a molecular level. ..
This lesson introduces the concept of electricity by asking students to imagine what their life would be like without electricity. Students learn that electrons can move between atoms, leaving atoms in a charged state.
Students are introduced to the concept of electricity by identifying it as an unseen, but pervasive and important presence in their lives. They compare conductors and insulators based on their capabilities for electron flow. Then water and electrical systems are compared as an analogy to electrical ..
Pre-Req Knowledge
Atom Molecule Image
Students should have some knowledge of atoms as the basic building blocks of matter.
Introduction/Motivation
Have you ever wondered what everything around you is made of? Are things made up of tiny particles that we cannot see? Yes, they are. These tiny building blocks of matter are called atoms and they make up everything we see around us, even ourselves. In this activity, we are going to learn about the atom. We will learn about the parts of an atom, its structure, and learn how to determine the charge of an atom. We will also learn how atoms come together to form molecules!
Molecules can be broken down into atoms, but can the atom actually be broken down into smaller parts? Yes, it can. Several subatomic particles make up an atom. The three main ones are protons and neutrons, which are found in the nucleus or core of the atom, and electrons, which are found floating around in shells outside of the nucleus. Physicists have recently divided atoms into even smaller subatomic particles such as fermions (quarks, leptons, neutrinos, electrons) and bosons (gluons, photons, gravitrons). It is difficult (if not impossible) to determine the physical properties of something based on the number or quarks and leptons it contains. The things we see in our world (water, wood, metal, skin, teeth) are better understood and organized by using the number of protons, neutrons and electrons their atoms (and molecules) contain. Just as we look at the shapes of different LEGO™ pieces, rather than the plastic that makes them, today we are just going to look at protons, neutrons and electrons as the 'LEGOs' of matter.
Did you know that atoms have energy? Well, these tiny subatomic particles are constantly moving or vibrating and we cannot even tell. For example, if you look at a molecule of water, it is made up of two hydrogen atoms and one oxygen atom. The electrons in each of these atoms that make up the water molecule are moving all around in their electron shells, or clouds. The outermost electrons in an atom are sometimes shared with another atom. That is how the molecule is formed. The large oxygen atom in a water molecule shares electrons with the hydrogen atoms.
How is this sharing possible? Well, it depends on the electrical charge of the atom. Each of our three subatomic particles in the atom has a type of charge. Protons have a positive charge or +1 charge on them. Electrons have a negative charge or a –1 charge to them. Neutrons are neutral or have no charge. When the overall charge of an atom is neutral, then there are an equal number of protons and electrons. If there are more protons than electrons, though, the atom is positively charged. If there are more electrons than protons, the atom is negatively charged. Any atom with a positive or negative overall charge is called an ion.
Do you think we can apply our knowledge of atoms and their structure to engineering? Yes! For example, how do engineers know that steel is extremely strong? Engineers know this because they know the characteristics of the atoms that make up steel. They know how the parts of each atom interact with each other to create a compound that has specific properties. Engineers and scientists have even been able to develop new atoms and elements that have specific properties that they need to create certain materials and technologies. Engineers use atoms, knowledge of their structure and how they bond, to make new medicines and products to help people. For example, environmental engineers need to know the properties of water molecules and the atoms that make up water molecules to design clean water and air treatment technologies to fight pollution.
Procedure
Voltei com ela matias damasio. Background
The goal of this activity is for students to understand the basic structure of an atom. This consists of the location and charge of protons, neutrons and electrons.Additionally, students will understand that molecules are made up of atoms in the same way that atoms are made up of protons, electrons and neutrons.
Students should be made aware of inaccuracies of the gumdrop model. Namely, the model does not correctly display the distances between the nucleus and electrons. Atoms are mostly empty space. For example, if the nucleus of a hydrogen atom were three miles wide and located in Kansas, its electrons would orbit near the East and West Coasts.
Before the Activity
- Gather all necessary materials.
- Make copies of the Atom Worksheet.
With the Students
- Divide the class into pairs and distribute one copy of the Atom Worksheet to each pair.
- Distribute gumdrops (4 red, 3 green, 3 blue/purple/white), toothpicks, skewers and sticker dots to each group.
- Instruct students to draw a '+' on three sticker dots, a '0' on four sticker dots, and a '-' on three sticker dots.
- Have students stick the '+'sticker dots to the 3 green gumdrops for protons, the '0' sticker dots on the 4 red gumdrops for neutrons, and the '-' sticker dots on the 3 blue/purple/white gumdrops to represent electrons. Explain that '+' means positive charge, '0' means no charge, and '-' means negative charge.
- Next, have students combine protons and neutrons in one cluster (using toothpicks broken in half) to form the nucleus. Ask what charge the nucleus has by itself and discuss why. (Answer: Positive; the nucleus is positive because it contains positive protons and neutral neutrons.)
- Then, have students place each electron on one end of a skewer, and stick the other end of the skewer in the nucleus to make a complete atom. Ask what charge the whole atom has and discuss why. (Answer: The atom has no charge because a negatively-charged electron balances out each positively-charged proton in the atom.)
- Instruct students to remove one electron from the atom. What is the charge of the atom now? (Answer: Positive. If an atom loses an electron (-), it becomes positively charged; because there are now more positive protons than negative electrons. If an atom picks up an electron (-), it becomes negatively charged; because there are now more negative electrons than positive protons. Electrically neutral atoms always have an equal number of electrons and protons, because the charges cancel each other out.)
- Now have the students get together with other students to join their own atoms to make molecules. If the elements are available, have the students move around the room and get together with other students to make known, simple molecules, such as water.
Note: Activity adapted from the Miami Museum of Science: http://www.miamisci.org/ph/lpexplain2.html
Assessment
Pre-Activity Assessment
Atom Detectives: Solicit, integrate and summarize student responses to the following questions.
- Can anyone name a specific type of atom? Can you find those types of atoms in the classroom? Have students name a few different atoms and where they might be found. For example, water contains oxygen and hydrogen.
Activity Embedded Assessment
Worksheet: Have students complete the Atom Worksheet. Review their answers to gauge their mastery of the subject.
Post-Activity Assessment Archicad 18 download with crack 64 bit.
What's the Charge? Engineers might need to know the electrical charge of an atom when designing new technologies. Have students determine the overall charge of the following atoms. Remind students that if there are more protons than electrons, then the atom is positively charged. If there are more electrons than protons, the atom is negatively charged. (Note: these ions may or may not occur naturally.)
- The atom has 6 protons, 8 neutrons and 6 electrons. (Answer: The charge is neutral. The atom is carbon-14.)
- The atom has 11 protons, 11 neutrons and 10 electrons. (Answer: The charge is positive. (+1) The atom is sodium.)
- The atom has 8 protons, 8 neutrons and 9 electrons. (Answer: The charge is negative (-1). The atom is oxygen.)
- The atom has 7 protons, 7 neutrons and 5 electrons. (Answer: The charge is positive (+2). The atom is nitrogen.)
- The atom has 29 protons, 29 neutrons and 29 electrons. (Answer: The charge is neutral. The atom is copper.)
Engineering Discussion: Ask a discussion question to get students to think about how engineering incorporates knowledge of atoms and atomic structure. How do engineers use the knowledge of the physical and electrical properties of different atoms? (Answer: Knowing what makes up an atom and how subatomic particles interact allows engineers to build stronger buildings, make more efficient medicine, and improve our daily lives through pollution control and consumer products.)
Safety Issues
Watch that students do not poke their neighbors with the toothpicks or skewers, as the fine filament structure of the wooden toothpicks and skewers can puncture the skin and leave nasty splinters.
Troubleshooting Tips
Remind the students that the gumdrops are for learning, not eating. It often helps to have an extra bag of gumdrops for the students to share and eat after the activity.
The students can use staples or glue to attach the sticker dots if they do not stick to the gumdrops.
Activity Extensions
Human Lithiium Atom: This activity demonstrates the huge amount of space between the nucleus and the electrons and dispels the common misconception that the electrons are located close to the nucleus. Have students make a human model of a lithium atom (same number of protons, neutrons and electrons as the gumdrop activity) on the playground or in the gymnasium. Students can wear T-shirts or caps of the same color, if possible, to represent protons, electrons and neutrons. Students can add pluses, minuses, or zeros, made out of electrical tape, to their shirts. Next, have students act in the roles of the protons, neutrons and electrons, with the 'nucleus' (protons and neutrons) as close to the center of the playground as possible and the electrons running around the edge of the playground. This human model is a little closer to scale, but still far different than the relative distances of an actual atom. In reality, if the nucleus formed by the students on the playground is about two feet wide (or a group hug made up of seven kids), then the electrons in the outside shell should actually be a mile away from the nucleus, not just at the edge of the playground!
References
Miami Museum of Science, The pH Factor, 'Atomic Gumdrops,' 2001, accessed August 31, 2006. http://www.miamisci.org
U.S. Department of Health and Human Services, GirlPower!, For Girls, 'Games and Puzzles,' accessed February 29, 2012. http://www.girlpower.com/
Copyright
© 2006 by Regents of the University of Colorado.Contributors
Brian Kay; Daria Kotys-Schwartz; Malinda Schaefer Zarske; Janet YowellSupporting Program
Integrated Teaching and Learning Program, College of Engineering, University of Colorado BoulderAcknowledgements
The contents of this digital library curriculum were developed under a grant from the Fund for the Improvement of Postsecondary Education (FIPSE), U.S. Department of Education, and National Science Foundation GK-12 grant no 0338326. However, these contents do not necessarily represent the policies of the Department of Education or National Science Foundation, and you should not assume endorsement by the federal government.
Last modified: January 23, 2021
Find more at TeachEngineering.org
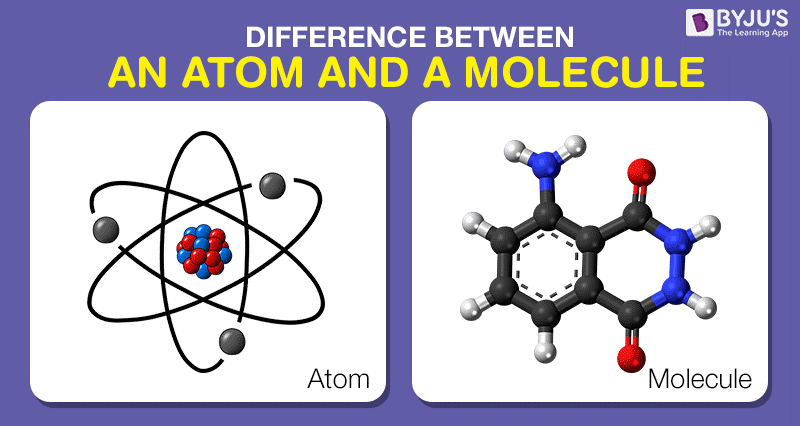
In your surrounding, you might have observed there are millions of objects, which are created out of matter and the matter comprises of molecules, which is a combination of two or more atoms bonded together. So, in short, atoms are grouped together to form a molecule, which embodies the objects around us. The whole universe is made up of the atom, and they are so small particles of an element, that we are not able to see them through bare eyes or even via microscope, but they do exist in each and every object and are affected by our activities.
What is the difference between atoms and molecules, is the basic question that arises in the mind of every chemistry student. They often face difficulty in understanding them correctly, as both of these two are a tiny identifiable unit.
Content: Atom Vs Molecule
Comparison Chart
| Basis for Comparison | Atom | Molecule |
|---|---|---|
| Meaning | The tiny particle of a chemical element, which may or may not exist independently is called an atom. | Molecules refers to the set of atoms held together by bond, indicating the smallest unit of a compound. |
| Existence | May or may not exist in free state. | Exist in free state. |
| Comprise of | Nucleus and electrons. | Two or more, identical or different atoms, bonded chemically. |
| Shape | Spherical | Linear, angular and triangular |
| Visibility | Neither visible through naked eyes, nor magnifying microscope. | Not visible through naked eyes, but can be seen with the help of magnifying microscope. |
| Reactivity | Highly reactive, subject to certain exceptions | Comparatively less reactive. |
| Bond | Nuclear bond | Covalent bond |
Definition of Atom
The term ‘atom’ in chemistry represents the elementary unit of ordinary matter that exist in free state and contains all chemical properties. It is an infinitesimal particle that distinctively identifies a chemical element. It is composed of a positively charged nucleus and surrounded by negatively charged electrons.
The nucleus consists of protons and neutrons which group together at the middle of an atom. These protons and neutrons have almost equal masses, but they vary in charge i.e. the former carry positive charge while the latter do not carry the electric charge. The positive charge in an atom is equivalent to the negative charge. Thus it is electrically neutral. In addition to this, the protons and neutrons are made up of constituents, i.e. quarks and gluons.
Atom Molecule Compound
Example: H, He, Li, O, N
Definition of Molecule
The molecule is a small unit of matter, which exist in free state and represents the chemical properties of the substance.
When two or more atoms are extremely close to each other, such that the electrons of the atoms can interact with one another, resulting in attraction between the atoms, called as a chemical bond. The chemical bond takes place as a result of the exchange of electrons between atoms, specifically known as a covalent bond. So, when two or more atoms are clustered together as a single unit, with the help of covalent bond, it forms a molecule.
Atom Molecule Order
If one or more identical atoms exist as a unit, independently, it is known as a molecule of an element, but if two or more varying elements are grouped together in a fixed proportion, by mass, to create a unit that exists freely, is termed as a molecule of a compound.
Example: H2O, CO2, NO2, CH4
Key Differences Between Atom and Molecule
The difference between atom and molecule can be drawn clearly on the following grounds:
- Atom is defined as the smallest unit of an element which may or may not exists independently. On the other hand, molecule implies the set of atoms held together by the bond, indicating the smallest unit of a compound.
- Atoms may or may not exist in the free state, but molecules exist in the free state.
- Atoms comprise of the nucleus (which contains protons and neutrons) and electrons. Conversely, a molecule comprises of two or more, identical or different atoms, combined chemically.
- The shape of an atom is spherical whereas the molecules can be linear, angular or rectangular in shape.
- Atoms are highly reactive, i.e. they participate in the chemical reaction without additional decomposition into subatomic units. However, this is not applicable to noble gas atoms. On the contrary, molecules are less reactive, as they do not take part in the chemical reaction.
- Atoms possess nuclear bond, as it entails electrostatic attraction between the nucleus and electron. In contrast, there exist a chemical bond between atoms of a molecule, such that it encompasses single, double or triple bonds.
Conclusion
Atom Molecule Ion
By and large, there are some dissimilarities amidst the two topics. Both are tiny units, but as molecules are made up of atoms, the size of an atom is much smaller than a molecule. Moreover, to form ions, atoms gain or lose electrons, which is not in the case of a molecule.
Atoms And Molecules Quizlet
Related Differences
You Might Also Like:
