- Positive Ion - Occurs when an atom loses an electron (negative charge) it has more protons than electrons. Negative Ion - Occurs when an atom gains an electron (negative charge) it will have more electrons than protons. The following image shows Na losing an electron and Cl gaining an electron. Thus the Na becomes Na+; The Cl becomes Cl.
- As Li-based systems, Na-based batteries come in different forms, such as Na-ion, Na-all-solid-state-batteries, NaO2 and Na/S. While the last ones are seen as disruptive future technologies, the Na.
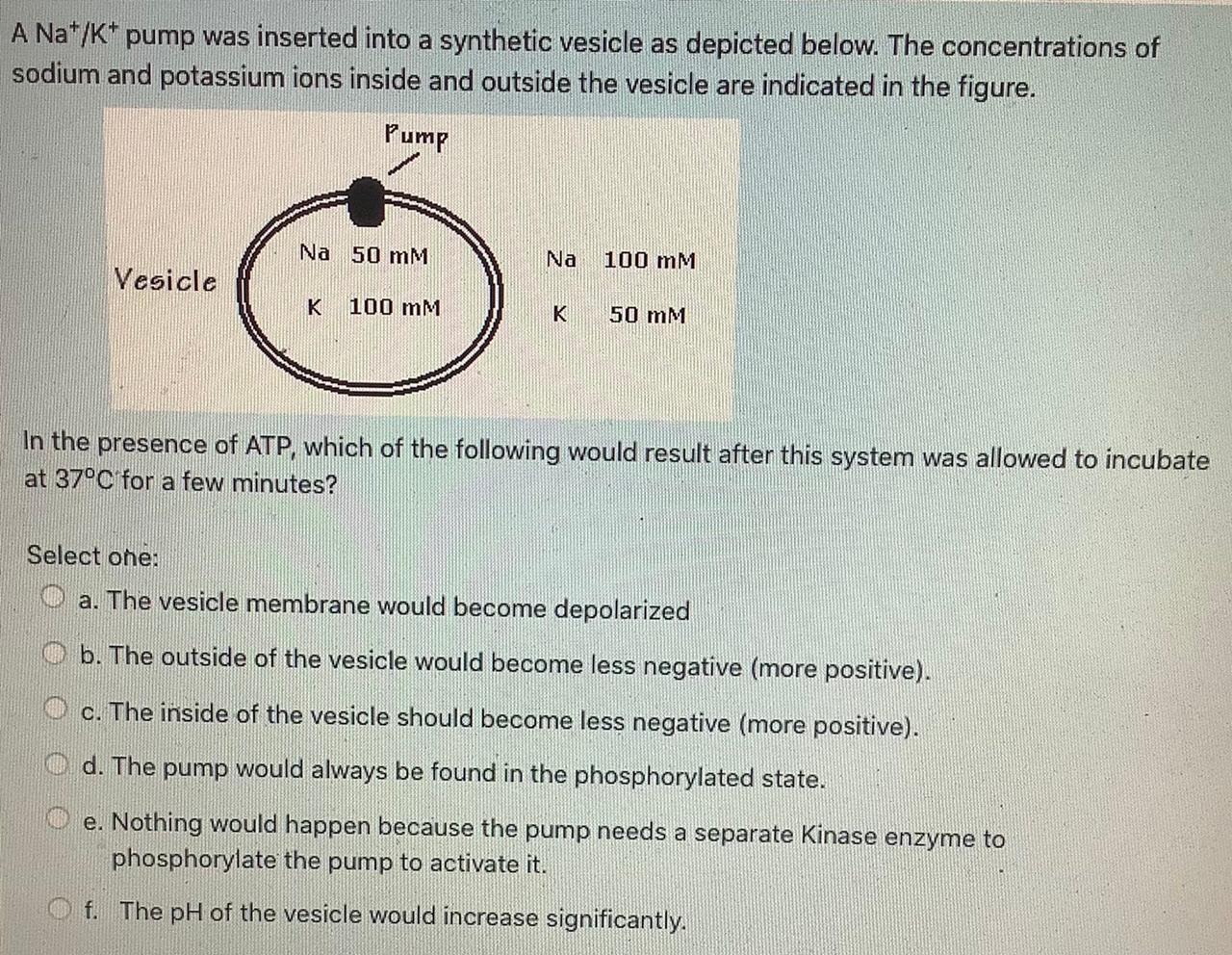
Sodium is a positively charged ion (Na+) and so it’s attracted to the negative polarity on the inside. As the positively charged sodium ions flow into the cell, the inside is going to become less negative (depolarized). So instead of -90mV, it’s going to go down to 0. Ion pairs occur in concentrated solutions of electrolytes (substances that conduct electricity when dissolved or molten). Thus, in concentrated solutions of sodium chloride, some positive sodium ions, Na +, and some negative chloride ions, Cl-, are paired together. Upon colliding, two oppositely charged ions stay together only for a short.
What is the electron configuration for a sodium ion?
1 Answer

The electron configuration of a neutral sodium atom is
In this configuration we note that there is only one electron in the 3rd energy level. Atoms prefer to gain the stability of octet, by having eight electrons in the outer shell, the electrons of the s and p orbitals. These are referred to as the valence orbitals and the valence electrons.
In the case of sodium the one lone electron in the 3s valence shell would easily be released in order for sodium to have a filled valence shell at
Therefore , the electron configuration of the sodium ion is
Because sodium gives up the electron from the 3s orbital it now has only 10 electrons but still has 11 protons, giving it a +1 charge and it becomes a
Ion Na+1
Related questions
